First an introction about oxidation. In the world of chemistry an oxidation reaction takes place, when substance that can be oxidated and some oxygen come in contact with each other. The forming of rust is a reaction of Iron and Oxygen and sped up by water and salt.In the case of tea leaves, the oxidation reaction is a reaction of polyphenols and oxygen accelarated by enzymes. The two enzyms most known to be responsible for this reaction are PolyPhenol Oxidase (PPO) and Peroxidase.
In living plants the different parts of the reaction are seperated from each other in the cells of the plant. From the moment a leaf is plucked, cell walls are damaged and all three chemicals can start reacting.
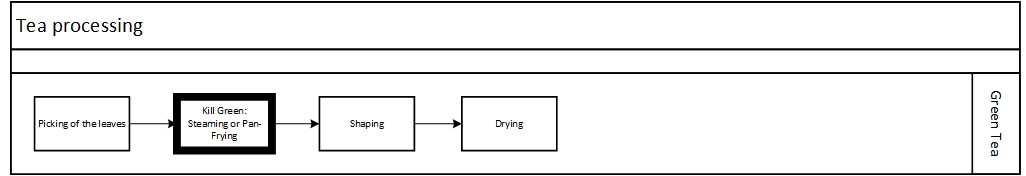
What tea manufacturers do to slow down this reaction, is the kill green method. They heat up the leaves to a temperature above 65°C of 150 °F to denaturize or break the enzym. This means that the reaction will slow down dramatically. Just like the forming of rust can take many years if iron doesnt get wet. If the enzyme is not present to speed up the reaction, it can take many years. This also explains the shelf life of green tea, your, green tea will not stay green for ever.


Does this mean that Japanese green tea is better? of course not, You might say that this is a choice made by the tradition and it is the tea masters choice which flavour balance he seeks.